Introduction to B cell repertoire analysis using the Immcantation framework¶

The field of high-throughput adaptive immune receptor repertoire sequencing (AIRR-seq) has experienced significant growth in recent years, but this growth has come with considerable complexity and variety in experimental design. These complexities, combined with the high germline and somatic diversity of immunoglobulin repertoires, present analytical challenges requiring specialized methodologies.
This tutorial covers:
- V(D)J gene annotation and novel polymorphism detection
- Inference of B cell clonal relationships
- Diversity analysis
- Mutational load profiling
- Modeling of somatic hypermutation (SHM) targeting
- Quantification of selection pressure
General workflow:
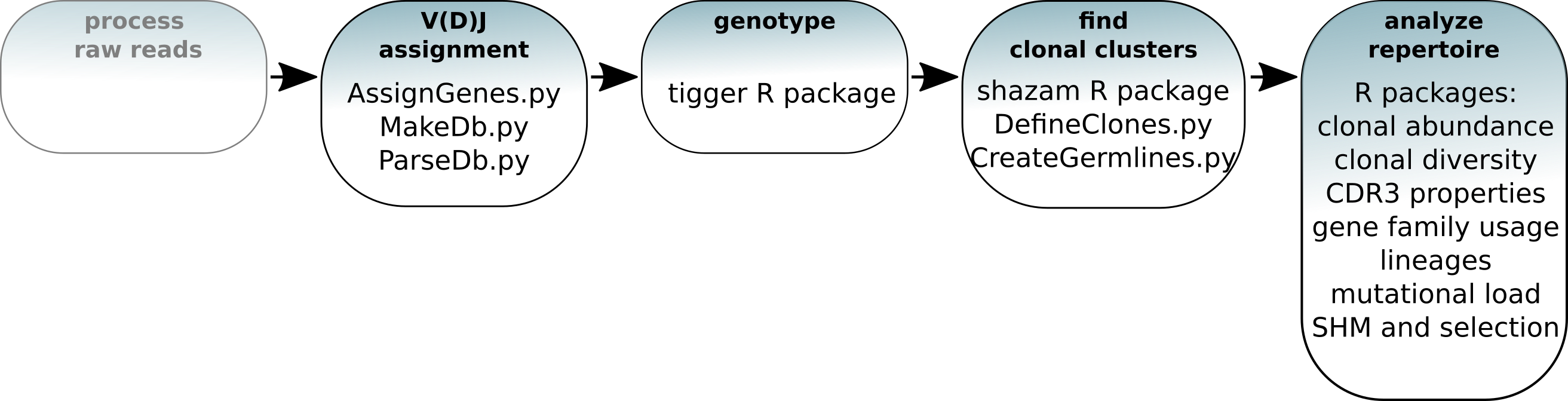
Resources¶
- You can email immcantation@googlegroups.com with any questions or issues.
- Documentation: http://immcantation.org
- Source code and bug reports: https://bitbucket.org/kleinstein/
- Docker/Singularity container for this lab: https://hub.docker.com/r/immcantation/lab
- Slides and example data: https://goo.gl/FpW3Sc
How to use the notebook¶
Jupyter Notebook documentation: https://jupyter-notebook.readthedocs.io/en/stable/
Ctrl+Enter will run the code in the selected cell and Shift+Enter will run the code and move to the following cell.
Inside this container¶
This container comes with software, scripts, reference V(D)J germline genes and example data that is ready to use. The commands versions report and builds report show the versions and dates respectively of the tools and data.
Software versions¶
Use this command to list the software versions
%%bash
versions report
Build versions¶
Use this command to list the date and changesets used during the image build.
%%bash
builds report
Example data used in the tutorial¶
../data/input.fasta: Processed B cell receptor reads from one healthy donor (PGP1) 3 weeks after flu vaccination (Laserson et al. (2014))
- As part of the processing, each sequence has been annotated with the isotype.
- This step is not a part of the tutorial, but you can learn how to do it here.
%%bash
head ../data/input.fasta
Reference germlines¶
The container has human and mouse reference V(D)J germline genes from IMGT and the corresponding IgBLAST databases for these germline repertoires.
%%bash
ls /usr/local/share/germlines/imgt
%%bash
ls /usr/local/share/igblast
V(D)J gene annotation¶
The first step in the analysis of processed reads (input.fasta) is to annotate each read with its germline V(D)J gene alleles and to identify relevant sequence structure such as the CDR3 sequence. Immcantation provides tools to read the output of many popular V(D)J assignment tools, including IMGT/HighV-QUEST and IgBLAST.
Here, we will use IgBLAST. Change-O provides a wrapper script (AssignGenes.py) to run IgBLAST using the reference V(D)J germline sequences in the container.
A test with 200 sequences¶
It is often useful to prototype analysis pipelines using a small subset of sequences. For a quick test of Change-O's V(D)J assignment tool, use SplitSeq.py to extract 200 sequences from input.fasta, then assign the V(D)J genes with AssignGenes.py.
%%bash
# reference germlines in /usr/local/share/igblast
mkdir -p results/igblast
SplitSeq.py sample -n 200 --outdir results --fasta -s ../data/input.fasta
AssignGenes.py performs V(D)J assignment with IgBLAST.
- It requires the input sequences (
-s) and a reference germlines database (-b). In this case, we use the germline database already available in the container. - The organism is specified with
--organismand the type of receptor with--loci(igfor the B cell receptor). - Use
--format blastto specify that the results should be in thefmt7format. - Improved computational speed can be achieved by specifying the number of processors with
--nproc.
%%bash
AssignGenes.py igblast -s results/input_sample1-n200.fasta \
-b /usr/local/share/igblast --organism human --loci ig \
--format blast --outdir results/igblast --nproc 8
V(D)J assignment using all the data¶
To run the command on all of the data, modify it to change the input file (-s) to the full data set. Please note that this may take some time to finish running.
%%bash
AssignGenes.py igblast -s ../data/input.fasta \
-b /usr/local/share/igblast --organism human --loci ig \
--format blast --outdir results/igblast --nproc 8
Data standardization using Change-O¶
Gupta NT, Vander Heiden JA, Uduman M, Gadala-Maria D, Yaari G, Kleinstein SH. Change-O: a toolkit for analyzing large-scale B cell immunoglobulin repertoire sequencing data. Bioinformatics 2015; doi: 10.1093/bioinformatics/btv359
Once the V(D)J annotation is finished, the IgBLAST results are parsed into a standardized format suitable for downstream analysis. All tools in the Immcantation framework use this format, which allows for interoperability and provides flexibility when designing complex workflows.
In this example analysis, the fmt7 results from IgBLAST are converted into the AIRR format, a tabulated text file with one sequence per row. Columns provide the annotation for each sequence using standard column names as described here: https://docs.airr-community.org/en/latest/datarep/rearrangements.html.
Generate a standardized database file¶
The command line tool MakeDb.py igblast requires the original input sequence fasta file (-s) that was passed to the V(D)J annotation tool, as well as the V(D)J annotation results (-i). The argument --format airr specifies that the results should be converted into the AIRR format. The path to the reference germlines is provided by -r.
%%bash
mkdir -p results/changeo
MakeDb.py igblast \
-s ../data/input.fasta -i results/igblast/input_igblast.fmt7 \
--format airr \
-r /usr/local/share/germlines/imgt/human/vdj/ --outdir results/changeo \
--outname data
Subset the data to include productive heavy chain sequences¶
We next filter the data from the previous step to include only productive sequences from the heavy chain. The determination of whether a sequence is productive (or not) is provided by the V(D)J annotation software.
ParseDb.py select finds the rows in the file -d for which the column productive (specified with -f) contains the values T or TRUE (specified by -u). The prefix data_p will be used in the name of the output file (specified by --outname).
%%bash
ParseDb.py select -d results/changeo/data_db-pass.tsv \
-f productive -u T --outname data_p
Next, we filter the data to include only heavy chain sequences.
ParseDb.py select finds the rows in the file -d from the previous step for which the column v_call (specified with -f) contains (pattern matching specified by --regex) the word IGHV (specified by -u). The prefix data_ph (standing for productive and heavy) will be used in the name of the output file (specified by --outname) to indicate that this file contains productive (p) heavy (h) chain sequence data.
%%bash
ParseDb.py select -d results/changeo/data_p_parse-select.tsv \
-f v_call -u IGHV --regex --outname data_ph
Set up the notebook to run R code¶
The following steps in the tutorial will use several R packages in the Immcantation framework. The next two lines of code are required to be able to use R magic and run R code in this Jupyter notebook.
# R for Python
import rpy2.rinterface
%load_ext rpy2.ipython
Genotyping and discovery of novel V gene alleles with TIgGER¶
Gadala-Maria D, Yaari G, Uduman M, Kleinstein S (2015). “Automated analysis of high-throughput B cell sequencing data reveals a high frequency of novel immunoglobulin V gene segment alleles.” Proceedings of the National Academy of Sciency of the United States of America, E862-70.
Gadala-Maria D, et al. (2019). “Identification of subject-specific immunoglobulin alleles from expressed repertoire sequencing data.” Frontiers in Immunology.
V(D)J assignment is a key step in analyzing repertoires and is done by matching the sequences against a database of known V(D)J alleles. However, current databases are incomplete and this process will fail for sequences that utilize previously undetected alleles. Some assignments may also occur to genes which are not carried by the individual. The TIgGER R package infers subject-specific V genotypes (including novel alleles), then uses the results to improve the V gene annotations.
Load libraries and read in the data¶
After loading the libraries needed, the process is started by using readChangeoDb to read the tabulated data generated in the previous step.
%%R
suppressPackageStartupMessages(library(airr))
suppressPackageStartupMessages(library(alakazam))
suppressPackageStartupMessages(library(dplyr))
suppressPackageStartupMessages(library(ggplot2))
suppressPackageStartupMessages(library(tigger))
dir.create(file.path("results","tigger"))
db <- read_rearrangement(file.path("results","changeo","data_ph_parse-select.tsv"))
colnames(db) # show the column names in the database
readIgFasta loads the reference V germline genes that were used in the V(D)J assignment (in this case the sequences in the Immcantation container).
%%R
ighv <- readIgFasta(file.path("","usr","local","share","germlines","imgt","human","vdj","imgt_human_IGHV.fasta"))
ighv[1] # show the first germline
Identify potentially novel V gene alleles¶
For each V gene allele, findNovelAlleles analyzes the sequences that were assigned the allele and evaluates the apparent mutation frequency at each position as a function of sequence-wide mutation counts. Positions that contain polymorphisms (rather than somatic hypermutations) will exhibit a high apparent mutation frequency even when the sequence-wide mutation count is low.
In this example, TIgGER finds one novel V gene allele.
%%R
nv <- findNovelAlleles(db, germline_db = ighv, nproc = 8) # find novel alleles
%%R
selectNovel(nv) # show novel alleles
The function plotNovel helps visualize the supporting evidence for calling the novel V gene allele. The mutation frequency of the position that is predicted to contain a polymorphism is highlighted in red. In this case, the position contains a high number of apparent mutations for all sequence-wide mutation counts (top panel).
By default, findNovelAlleles uses several additional filters to avoid false positive allele calls. For example, to avoid calling novel alleles resulting from clonally-related sequences, TIgGER requires that sequences perfectly matching the potential novel allele be found in sequences with a diverse set of J genes and a range of junction lengths. This can be observed in the bottom panel.
%%R
plotNovel(db, selectNovel(nv)[1,]) # visualize the novel allele(s)
Genotyping, including novel V gene alleles¶
The next step is to infer the subject’s genotype and use this to improve the V gene calls.
inferGenotype uses a frequency-based method to determine the genotype. For each V gene, it finds the minimum set of alleles that can explain a specified fraction of each gene’s calls. The most commonly observed allele of each gene is included in the genotype first, then the next most common allele is added until the desired fraction of sequence annotations can be explained.
Immcantation also includes other methods for inferring subject-specific genotypes (inferGenotypeBayesian).
%%R
gt <- inferGenotype(db, germline_db = ighv, novel = nv)
# save genotype inf .fasta format to be used later with CreateGermlines.py
gtseq <- genotypeFasta(gt, ighv, nv)
writeFasta(gtseq, file.path("results","tigger","v_genotype.fasta"))
gt %>% arrange(total) %>% slice(1:3) # show the first 3 rows
The individual's genotype can be visualized with plotGenotype. Each row is a gene, with colored cells indicating each of the alleles for that gene that are included in the inferred genotype.
%%R
plotGenotype(gt) # genotyping including novel V gene alleles
Reassign V gene allele annotations¶
Some of the V gene calls may use genes that are not part of the subject-specific genotype inferred by TIgGER. These gene calls can be corrected with reassignAlleles. Mistaken allele calls can arise, for example, from sequences which by chance have been mutated to look like another allele. reassignAlleles saves the corrected calls in a new column, v_call_genotyped.
With write_airr the updated data is saved as a tabulated file for use in the following steps.
%%R
db <- reassignAlleles(db, gtseq)
# show some of the corrected gene calls
db %>% filter(v_call != v_call_genotyped) %>% sample_n(3) %>% select(v_call, v_call_genotyped)
%%R
write_airr(db, file.path("results","tigger","data_ph_genotyped.tsv"))
Clonal diversity analysis¶
Goal: Partition (cluster) sequences into clonally related lineages. Each lineage is a group of sequences that came from the same original naive cell.
Summary of the key steps:
- Determine clonal clustering threshold: sequences which are under this cut-off are clonally related.
- Assign clonal groups: add an annotation (
clone_id) that can be used to identify a group of sequences that came from the same original naive cell. - Reconstruct germline sequences: figure out the germline sequence of the common ancestor, before mutations are introduced during clonal expansion and SMH.
- Analize clonal diversity: number and size of clones? any expanded clones?
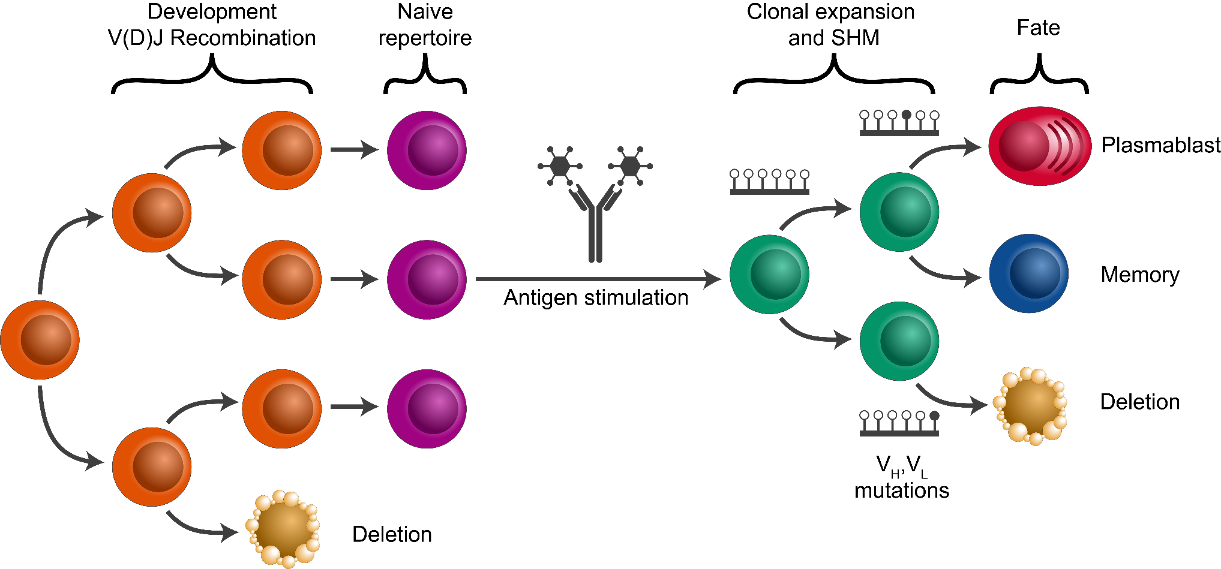
Clonal assignment using the CDR3 as a fingerprint¶
Gupta et al. (2017)
Nouri and Steven H Kleinstein (2018b)
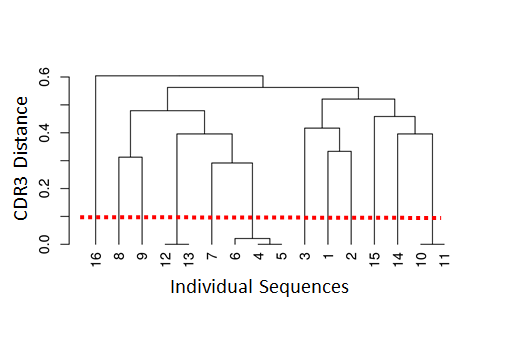
Clonal relationships can be inferred from sequencing data. Hierarchical clustering is a widely used method for identify clonally related sequences. This method requires a measure of distance between pairs of sequences and a choice of linkage to define the distance between groups of sequences. Since the result will be a tree, a threshold to cut the hierarchy into discrete clonal groups is also needed.
The figure below on the left shows a tree and the chosen threshold (red dotted line). Sequences which have a distance under this threshold are considered to be part of the same clone (i.e., clonally-related) whereas sequences which have distance above this threshold are considered to be part of independent clones (i.e., not clonally related).
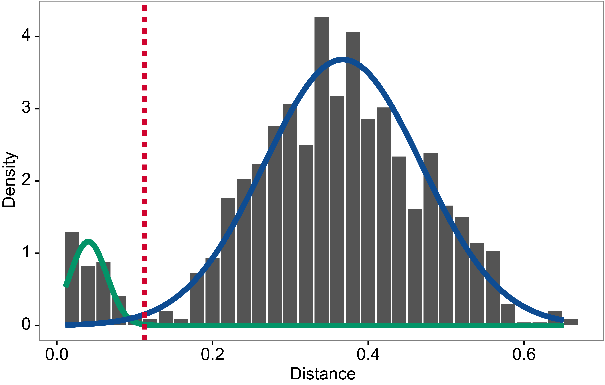
shazam provides methods to calculate the distance between sequences and find an appropriate distance threshold for each dataset (distToNearest and findThreshold). The threshold can be determined by analyzing the distance to the nearest distribution. This is the set of distances between each sequence and its closest non-identical neighbour.
The figure below on the right shows the distance-to-nearest distribution for a repertoire. Typically, the distribution is bimodal. The first mode (on the left) represents sequences that have at least one clonal relative in the dataset, while the second mode (on the right) is representative of the sequences that do not have any clonal relatives in the data (sometimes called "singletons"). A reasonable threshold will separate these two modes of the distribution. In this case, it is easy to manually determine a threshold as a value intermediate between the two modes. However, findThreshold can be used to automatically find the threshold.
Setting the clonal distance threshold with SHazaM¶
Gupta et al. (2015)
We first split sequences into groups that share the same V and J gene assignments and that have the same junction (or equivalently CDR3) length. This is based on the assumption that members of a clone will share all of these properties. distToNearest performs this grouping step, then counts the number of mismatches in the junction region between all pairs of sequences in eaach group and returns the smallest non-zero value for each sequence. At the end of this step, a new column (dist_nearest) which contains the distances to the closest non-identical sequence in each group will be added to db.
findThreshold uses the distribution of distances calculated in the previous step to determine an appropriate threshold for the dataset. This can be done using either a density or mixture based method. The function plot can be used to visualize the distance-to-nearest distribution and the threshold.
%%R -o thr
# get the distance to nearest neighbors
suppressPackageStartupMessages(library(shazam))
db <- distToNearest(db, model = "ham", normalize = "len", vCallColumn = "v_call_genotyped", nproc = 4)
# determine the threshold
threshold <- findThreshold(db$dist_nearest, method = "density")
thr <- round(threshold@threshold, 2)
thr
%%R
plot(threshold) # plot the distribution
Advanced method: Spectral-based clonal clustering (SCOPer)¶
Nouri and Steven H Kleinstein (2018a)
In some datasets, the distance-to-nearest distribution may not be bimodal and findThreshold may fail to determine the distance threshold. In these cases, spectral clustering with an adaptive threshold to determine the local sequence neighborhood may be used. This can be done with functions from the SCOPer R package.
Change-O: Clonal assignment¶
Once a threshold is decided, the command line tool DefineClones.py from Change-O performs the clonal assignment. There are some arguments that need to be specified:
--model: the distance metric used indistToNearest--norm: type of normalization used indistToNearest--dist: the cut-off fromfindThreshold
At the end of this step, the output file will have an additional column (clone_id) that provides an identifier for each sequence to indicate which clone it belongs to (i.e., sequences that have the same identifier are clonally-related). Note that these identifiers are only unique to the dataset used to carry out the clonal assignments.
# assign clonal groups
! DefineClones.py -d results/tigger/data_ph_genotyped.tsv --vf v_call_genotyped \
--model ham --norm len --dist {thr[0]} --format airr --nproc 8 \
--outname data_ph_genotyped --outdir results/changeo/
Change-O: add full length germline sequences¶
The next step is to identify the V(D)J germline sequences from which each of the observed sequences is derived. These germlines will be used in downstream analysis to infer somatic mutations and reconstruct lineages. CreatGermlines.py takes the alignment information in the Change-O file (-d) as well as the reference database used by the alignment software (-r) and generates a germline sequence for each individual observed sequence.
If the argument --cloned is used, the function assigns the same germline to all sequences belonging to the same clone. -g is used to specify the type of germlines to be reconstructed. In this example, -g dmask is used to mask the D region, meaning that all nucleotides in the N/P and D-segments are replaced with N's. This is often done because the germline base calls from this region are unreliable for B cell receptor alignments.
At the end of this step, the data file will have the germline sequence in the germline_alignment_d_mask column.
%%bash
CreateGermlines.py -d results/changeo/data_ph_genotyped_clone-pass.tsv \
-r /usr/local/share/germlines/imgt/human/vdj/*IGH[DJ].fasta results/tigger/v_genotype.fasta \
-g dmask --cloned --vf v_call_genotyped \
--format airr --outname data_ph_genotyped
Alakazam: Analysis of clonal diversity¶
Gupta et al. (2015)
Once the database is annotated with clonal relationships (clone_id), the R package alakazam is used to analyze clonal diversity and abundance.
Load data and subset to IGHM, IGHG and IGHA¶
The tab-delimited file is loaded with the function read_rearrangement. In this example, the analysis will focus separately on sequences with different isotypes.
- The isotype for each sequences (IGHM, IGHG or IGHA) is in the
isotypecolumn. - The function
subsetis used to keep only those sequences as part of the analysis.
%%R
# update names in alakazam’s default colors (IG_COLORS)
names(IG_COLORS) <- c( "IGHA", "IGHD", "IGHE", "IGHG", "IGHM","IGHK", "IGHL")
# read in the file that passed cloning and germline creation
db <- read_rearrangement(file.path("results", "changeo", "data_ph_genotyped_germ-pass.tsv"))
# subset data to IgA, IgG and IgM
db <- subset(db, isotype %in% c("IGHA", "IGHG", "IGHM"))
Clonal abundance¶
Clonal abundance is the size of each clone (as a fraction of the entire repertoire). estimateAbundance estimates the clonal abundance distribution along with confidence intervals on these clone sizes using bootstrapping.
In this example, the abundance is analyzed by isotype (group = "isotype"). In the figure below, the y-axis shows the clone abundance (i.e., the size as a percent of the repertoire) and the x-axis is a rank of each clone, where the rank is sorted by size from larger (rank 1, left) to smaller (right). The shaded areas are confidence intervals.
%%R
# calculate the rank-abundance curve
a <- estimateAbundance(db, group = "isotype")
plot(a, colors = IG_COLORS)
Clonal diversity¶
The clonal abundance distribution can be characterized using diversity statistics. Diversity scores (D) are calculated using the generalized diversity index (Hill numbers), which covers many different measures of diversity in a single function with a single varying parameter, the diversity order q.
The function alphaDiversity resamples the sequences and calculates diversity scores (D) over a interval of diversity orders (q). The diversity (D) is shown on the y-axis and the x-axis is the parameter q.
q = 0corresponds to Species Richnessq = 1corresponds to Shannon Entropyq = 2corresponds to Simpson Index
Inspection of this figure is useful to determine whether any difference in diversity between two repertoires depends on the statistic used or if it is a universal property. For example, you can see that the blue curve is changing when you change q, whereas the diversity of the green and red curves doesn't change significantly along values of q.
%%R
# generate the Hill diversity curve
d <- alphaDiversity(db, group = "isotype")
p <- plot(d, silent = T)
p + geom_vline(xintercept = c(0,1,2), color = "grey50", linetype = "dashed") +
geom_text(data = data.frame(q = c(0,1,2), y = round(max(p$data$d_upper)/2),
label = c("Richness", "Shannon", "Simpson")),
aes(x = q, y = y,label = label), size = 3, angle = 90, vjust = -0.4, inherit.aes = F, color = "grey50")
Alakazam: Physicochemical properties of the CDR3¶
CDR3 is the most variable region of the antibody sequence and its physicochemical properties are key contributors to antigen specificity. The function aminoAcidProperties in the alakazam R package can calculate several amino acid sequence physicochemical properties such as length, hydrophobicity (GRAVY index), bulkiness, polarity and net charge, among others. To obtain the CDR3, the junction sequence (specified by seq = "junction"), which is available in the form of a nucleotide sequence (nt = T), is trimmed to remove the first and last codon/amino acids before calculating the properties.
The figure below, generated with standard ggplot commands, shows the distribution of the CDR3 amino acid length by isotype.
%%R
# calculate CDR3 amino acid properties
db <- aminoAcidProperties(db, seq = "junction", nt = T, trim = T, label = "cdr3")
# Plot
ggplot(db, aes(x = isotype, y = cdr3_aa_length)) + theme_bw() +
ggtitle("CDR3 length") + xlab("Isotype") + ylab("Amino acids") +
scale_fill_manual(name = "Isotype", values = IG_COLORS) +
geom_boxplot(aes(fill = isotype))
Alakazam: family, gene and allele usage¶
The study of biases in gene usage is also of interest. The function countGenes from the alakazam R packages can determine the count and relative abundance of V, D and J at the level of alleles, genes, or families.
V family usage by isotype¶
Here, countGenes counts V gene family usage (mode = "family") by isotype (groups = "isotype") using the V gene allele calls in the column v_call_genotyped. --clone specifies each clone will be considered only once, with the most common gene within the clone being used.
%%R
# V family usage by isotype
# "clone" specifies to consider one gene per clone_id,
# the most common gene within each clone
usage_fam_iso <- countGenes(db, gene = "v_call_genotyped", groups = "isotype", clone = "clone_id", mode = "family")
# groups = "isotype", then usage by isotype sums 1
usage_fam_iso %>% group_by(isotype) %>% summarize(total = sum(clone_freq))
%%R
ggplot(usage_fam_iso, aes(x = isotype, y = clone_freq)) +
geom_point(aes(color = isotype), size = 4) +
scale_fill_manual(name = "Isotype", values = IG_COLORS) +
facet_wrap(~gene, nrow = 1) +
theme_bw() + ggtitle("V Family usage by isotype") +
ylab("Percent of repertoire") + xlab("Isotype")
Alakazam: lineage reconstruction¶
Hoehn et al. (2019)
B cell repertoires often consist of hundreds to thousands of separate clones. A clonal lineage recapitulates the ancestor-descendant relationships between clonally-related B cells and uncovering these relationships can provide insights into affinity maturation. The R package alakazam uses PHYLIP to reconstruct lineages following a maximum parsimony technique. PHYLIP is already installed in the Immcantation container.
Before performing lineage reconstruction, some preprocessing in needed. The code below shows an example of such preprocessing done for one of the largest clones in the example dataset. The function makeChangeoClone takes as input a data.frame with information for a clone (db_clone). text_fields = "isotype" specifies that annotation in the column isotype should be merged during duplicate removal. For example, if two duplicate sequences (defined by identical nucleotide composition) are found, and one is annotated as IGHM and the second one is an IGHG, then they will be "collapsed" into a single sequence that will have the isotype value "IGHM,IGHG". The preprocessing done by makeChangeoClone also includes masking gap positions and masking ragged ends.
%%R
# Select one clone, the 2nd largest, just as an example
largest_clone <- countClones(db) %>% slice(2) %>% select(clone_id) %>% as.character()
# Subset db, get db with data for largest_clone
db_clone <- subset(db, clone_id == largest_clone)
# Build tree from a single clone
x <- makeChangeoClone(db_clone, v_call = "v_call_genotyped", text_fields = "isotype")
Lineage reconstruction is done with buildPhylipLineage. This function uses the dnapars tool from PHYLIP, which in the container is located at /usr/local/bin/dnapars, to build the lineage, and returns an igraph object. This object can be quickly visualized with the command plot. It is possible to use igraph or other tools to resize nodes (e.g., by the underlying sequence count) or add colors (e.g., to indicate tissue location of B cell subsets) to help answer specific biological questions.
%%R
# Lineage reconstruction
g <- buildPhylipLineage(x, phylip_exec = "/usr/local/bin/dnapars")
suppressPackageStartupMessages(library(igraph))
plot(g)
Alakazam: lineage topology analysis¶
alakazam has several functions to analyze the reconstructed lineage. getMRCA retrieves the first non-root node of a lineage tree, which is the Most Recent Common Ancestor (MRCA) for all the sequences in the clone. getpathlength calculates all the path lengths (in terms of the number of point mutations) from the tree root to all individual sequences. summarizeSubtrees calculates summary statistics for each node of a tree (node name, name of the parent node, number of edges leading from the node, total number of nodes within the subtree rooted at the node...). tableEdges creates a table of the total number of connections (edges) for each unique pair of annotations within a tree over all nodes.
%%R
# Retrieve the most ancestral sequence
getMRCA(g, root="Germline")
%%R
# Calculate distance from germline
getPathLengths(g, root="Germline") %>% top_n(2)
%%R
# Calculate subtree properties
summarizeSubtrees(g, fields="isotype") %>% top_n(2)
%%R
# Tabulate isotype edge relationships
tableEdges(g, "isotype", exclude=c("Germline", NA))
SHazaM: mutational load¶
B cell repertoires differ in the number of mutations introduced during somatic hypermutation (SHM). The shazam R package provides a wide array of methods focused on the analysis of SHM, including the reconstruction of SHM targeting models and quantification of selection pressure.
Having identified the germline sequence (germline_alignment_d_mask) in previous steps, we first identify the set of somatic hypermutations by comparing the observed sequence (sequence_alignment) to the germline sequence. observedMutations is next used to quantify the mutational load of each sequence using either absolute counts (frequency=F) or as a frequency (the number of mutations divided by the number of informative positions (frequency=T). Each mutation can be defined as either a replacement mutation (R, or non-synonymous mutation, which changes the amino acid sequence) or a silent mutation (S, or synonymous mutation, which does not change the amino acid sequence). R and S mutations can be counted together (conmbine=T) or independently (combine=F). Counting can be limited to mutations occurring within a particular region of the sequence (for example, to focus on the V region regionDefinition=IMGT_V) or use the whole sequence (regionDefinition=NULL).
Standard ggplot commands can be used to generate boxplots to visualize the distribution of mutation counts across sequences, which are found in the column mu_count that observedMutations added to db.
%%R
# Calculate total mutation count, R and S combined
db <- observedMutations(db,
sequenceColumn="sequence_alignment",
germlineColumn="germline_alignment_d_mask",
regionDefinition=NULL, frequency=F, combine = T, nproc=4)
ggplot(db, aes(x=isotype, y=mu_count, fill=isotype)) +
geom_boxplot() +
scale_fill_manual(name="Isotype",values=IG_COLORS) +
xlab("Isotype") + ylab("Mutation count") + theme_bw()
SHazaM: models of SHM targeting biases¶
Yaari et al. (2013)
SHM is a stochastic process, but does not occur uniformly across the V(D)J sequence. Some nucleotide motifs (i.e., hot-spots) are targeted more frequently than others (i.e., cold-spots). These intrinsic biases are modeled using 5-mer nucleotide motifs. For each 5-mer (e.g., ATCTA), a SHM mutability model provides the relative likelihood for the center base (e.g., the C in ATCTA) to be mutated. As associated substitution model provides the relative probabilities of the center base in a 5-mer mutating to each of the other 3 bases. There are several pre-built SHM targeting models included in shazam which have been constructed based on experimental datasets. These include models for human and mouse, and for both light and heavy chains. In addition, shazam provides the createTargetingModel method to generate new SHM targeting models from user-supplied sequencing data.
 The resulting model can be visualized as a hedgehog plot with
The resulting model can be visualized as a hedgehog plot with plotMutability. In this visualization, 5-mers that belong to classically-defined SHM hot-spot motifs (e.g., WRC) are shown in red and green, cold-spot motifs in blue, and other motifs (i.e., neutral) in grey. The bars radiating outward are the relative mutabilities of each 5-mer. The middle circle is the central nucleotide being targeted for SHM, here shown by a green circle on the left plot (nucleotide A) and an orange circle on the right plot (nucleotide C). Moving from the center of the circle outward covers the 5-mer nucleotide motif (5' to 3'). As expected, higher relative mutation rates are observed at hot-spot motifs.
%%R
# Build and plot SHM targeting model
m <- createTargetingModel(db, vCallColumn="v_call_genotyped")
# nucleotides: center nucleotide characters to plot
plotMutability(m, nucleotides=c("A","C"), size=1.2)
SHazaM: quantification of selection pressure¶
Yaari et al. (2012)
During T-cell dependent adaptive immune responses, B cells undergo cycles of SHM and affinity-dependent selection that leads to the accumulatation of mutations that improve their ability to bind antigens. The ability to estimate selection pressure from sequence data is of interest to understand the events driving physiological and pathological immune responses. shazam incorporates the Bayesian estimation of Antigen-driven SELectIoN (BASELINe) framework, to detect and quantify selection pressure, based on the analysis of somatic hypermutation patterns. Briefly, calcBaseline analyzes the observed frequency of replacement and silent mutations normalized by their expectated frequency based on a targeting model, and generates a probability density function (PDF) for the selection strength ($\sum$). This PDF can be used to statistically test for the occurrence of selection, as well as compare selection strength across conditions. An increased frequency of R mutations ($\sum>0$) suggests positive selection, and a decreased frequency of R mutations ($\sum<0$) points towards negative selection .
The somatic hypermutations that are observed in clonally-related sequences are not independent events. In order to generate a set of independent SHM events, selection pressure analysis starts by collapsing each clone to one representative sequence (with its associated germline) using the function collapseClones. Next calcBaseline takes each representative sequences and calculates the selection pressure. The analysis can be focused on a particular region, the V region (regionDefinition=IMGT_V) in the example below. Because selection pressure may act differently across the BCR, we may also be interested to evaluate the selection strength for different regions such as the Complementary Determining Regions (CDR) or Framework Regions (FWR) separately. Selection strength can also be calculated separately for different isotopes or subjects. This can be done with groupbaseline, which convolves the PDFs from individual sequences into a single PDF representing the group of sequences (e.g., all IgG sequences).
These PDF can be visualized with plot. FWR are critical to maintenance the overall structure of the BCR and typically have a lower observed selection strength, because mutations that alter the amino acid sequence are often negatively selected. CDRs are the areas of the BCR that generally bind to the antigen and may be subject to increased selection strength, although this can depend on the point in affinity maturation when the sequence is observed. In the example, negative selection is observed in the FWR, and almost no selection is observed in the CDR. In both CDR and FWR, sequences annotated with IGHM isotype show more positive selection strength than sequences annotated with IGHG and IGHA.
%%R
# Calculate clonal consensus and selection using the BASELINe method
z <- collapseClones(db)
b <- calcBaseline(z, regionDefinition=IMGT_V)
# Combine selection scores for all clones in each group
g <- groupBaseline(b, groupBy="isotype")
# Plot probability densities for the selection pressure
plot(g, "isotype", sigmaLimits=c(-1, 1), silent=F)
SHazaM: Built in mutation models¶
shazam comes with serveral pre-built targeting models, mutation models and sequence region definitions. They can be used to change the default behaviour of many functions. For example, mutations are usually defined as R when they introduce any change in the amino acid sequence (NULL). However, for some analyses, it may be useful to consider some amino acid substitutions to be equivalent (i.e., treated as silent mutations). For example, it is possible to define replacement mutations as only those that introduce a change in the amino acid side chain charge (CHARGE_MUTATIONS).
Empirical SHM Targeting Models
HH_S5F: Human heavy chain 5-mer model
HKL_S5F: Human light chain 5-mer model
MK_RS5NF: Mouse light chain 5-mer model
Mutation Definitions
NULL: Any mutation that results in an amino acid substitution is considered a replacement (R) mutation.
CHARGE_MUTATIONS: Only mutations that alter side chain charge are replacements.
HYDOPATHY_MUTATIONS: Only mutations that alter hydrophobicity class are replacements.
POLARITY_MUTATIONS: Only mutations that alter polarity are replacements.
VOLUME_MUTATIONS: Only mutations that alter volume class are replacements.
Sequence Region Definitions
NULL: Full sequence
IMGT_V: V segment broken into combined CDRs and FWRs
IMGT_V_BY_REGIONS: V segment broken into individual CDRs and FWRs
References¶
Gadala-Maria,D. et al. (2019) Identification of subject-specific immunoglobulin alleles from expressed repertoire sequencing data. Frontiers in Immunology, 10, 129.
Gadala-Maria,D. et al. (2015) Automated analysis of high-throughput b-cell sequencing data reveals a high frequency of novel immunoglobulin v gene segment alleles. Proceedings of the National Academy of Sciences, 201417683.
Gupta,N.T. et al. (2017) Hierarchical clustering can identify b cell clones with high confidence in ig repertoire sequencing data. The Journal of Immunology, 1601850.
Gupta,N.T. et al. (2015) Change-o: A toolkit for analyzing large-scale b cell immunoglobulin repertoire sequencing data. Bioinformatics, 31, 3356–3358.
Hoehn, K. B. et al. (2019) Repertoire-wide phylogenetic models of B cell molecular evolution reveal evolutionary signatures of aging and vaccination. PNAS 201906020.
Laserson,U. et al. (2014) High-resolution antibody dynamics of vaccine-induced immune responses. Proc. Natl. Acad. Sci. U.S.A., 111, 4928–4933.
Nouri,N. and Kleinstein,S.H. (2018a) A spectral clustering-based method for identifying clones from high-throughput b cell repertoire sequencing data. Bioinformatics, 34, i341–i349.
Nouri,N. and Kleinstein,S.H. (2018b) Optimized threshold inference for partitioning of clones from high-throughput b cell repertoire sequencing data. Frontiers in immunology, 9.
Stern,J.N. et al. (2014) B cells populating the multiple sclerosis brain mature in the draining cervical lymph nodes. Science translational medicine, 6, 248ra107–248ra107.
Vander Heiden,J.A. et al. (2017) Dysregulation of b cell repertoire formation in myasthenia gravis patients revealed through deep sequencing. The Journal of Immunology, 1601415.
Yaari,G. et al. (2012) Quantifying selection in high-throughput immunoglobulin sequencing data sets. Nucleic acids research, 40, e134–e134.
Yaari,G. et al. (2013) Models of somatic hypermutation targeting and substitution based on synonymous mutations from high-throughput immunoglobulin sequencing data. Frontiers in immunology, 4, 358.